Фазовая диаграмма системы Hf-Ni
К оглавлению: Другие диаграммы (Others phase diargams)
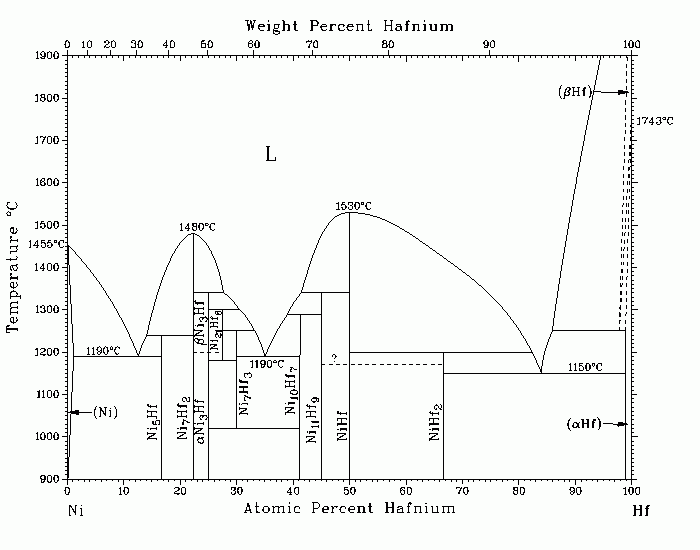
Hf-Ni (Hafnium-Nickel) P. Nash and A. Nash The Ni-Hf system is complex because of the number of intermediate phases formed: Ni5Hf, Ni7Hf2, Ni3Hf, Ni21Hf8, Ni7Hf3, Ni10Hf7, Ni11Hf9, NiHf, and NiHf2. It is very similar to the Ni-Zr system. Ni7Hf2 and NiHf are congruently melting, whereas the other seven phases are formed peritectically. No information is available regarding solubility in the intermediate phases; consequently, these are assumed to be line compounds. The assessed phase diagram is based primarily on the work of [79Bse] and [67Sve], with a review of the work of [61Kir]. In the central portion of the diagram, [79Bse] reported that Ni7Hf3 exists with a limited range of stability from 1250 с 20 to 1016 с 3 C, at which temperature the phase decomposes to Ni10Hf7 and bBi3Hf. [67Sve] obtained thermal arrests in the solid state around the composition NiHf, which they attributed to a polymorphic transition in that compound. Although limited data are available on the temperature variation of the solid solubility of Hf in (Ni) and of Ni in (Hf), temperatures are very restricted. The maximum solubility of Hf in (Ni) is 1 at.% Hf at 1190 C. The solubility ranges of the Ni-rich and Hf-rich terminal solutions are very restricted. In Ni1-xHfx amorphous alloys prepared by arc melting followed by melt spinning in an atmosphere of purified argon for the concentration range 0.11 њ x њ 0.80, amorphous structures were observed for the following compositions: Ni20Hf80, Ni38Hf62, Ni64Hf36, and Ni89Hf11. 61Kir: M.E. Kirkpatrick and W.L. Larsen, Trans. ASM, 54, 580-590; discussion, 851-853 (1961). 67Sve: V.N. Svechnikov, A.K. Shurin, and G.P. Dmitriyeva, Russ. Metall., (6), 95-96 (1967). 79Bse: L. Bsenko, J. Less-Common Met., 63, 171-179 (1979). Published in Phase Diagrams of Binary Nickel Alloys, 1991. Complete evaluation contains 1 figure, 2 tables, and 23 references. Special Points of the Ni-Hf System
