Фазовая диаграмма системы Al-Mg
К оглавлению: Другие диаграммы (Others phase diargams)
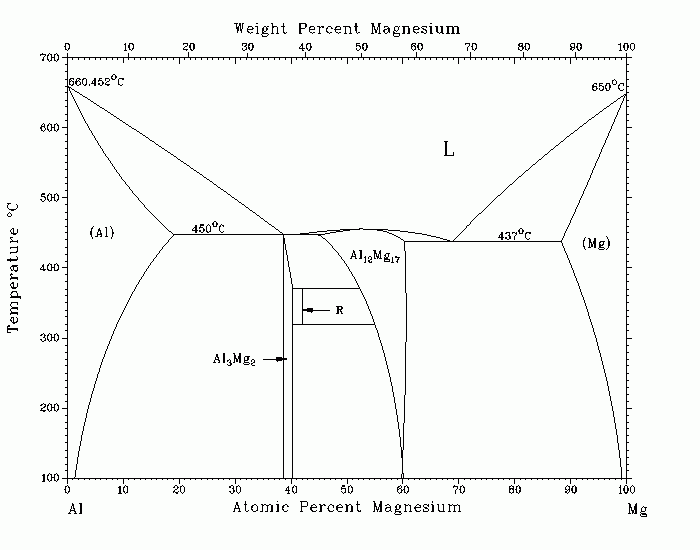
Al-Mg (Aluminum-Magnesium) J.L. Murray The equilibrium solid phases of the Al-Mg system are (1) the fcc (Al) solid solution, with a maximum solubility of Mg in (Al) of 18.9 at.% at a eutectic temperature of 450 C; (2) the cph (Mg) solid solution, with a maximum solubility of Al in (Mg) of 11.8 at.% at a eutectic temperature of 437 C; (3) the b compound of approximate stoichiometry Al3Mg2, with a complex fcc structure (at low temperature, b transforms martensitically to another structure that may be a distortion of the b structure, but the equilibrium phase relations have not been investigated); (4) the line compound R (often designated e), of composition 42 at.% Mg; and (5) the compound g, with the aMn structure (at 450 C, g has a maximum composition range of approximately 45 to 60.5 at.% Mg, but the ideal crystal structure has the stoichiometry Al12Mg17 at 58.6 at.% Mg). The phase boundaries in the assessed phase diagram were obtained from thermodynamic calculations, with the exception of the single-phase b field. For the b phase, a line compound was used in the calculations, although b is known to exist over a range of composition. The present diagram is based on a review of the work of [33Sch], [35Hau], [35Zak], [38Hum], [38Kur], [39Sie], [ 45But], [70Ban], and [79Sti]. Supersaturated (Al) solid solutions are readily obtained, and decomposition proceeds by the formation of spherical GP zones. A possible spinodal ordering mechanism has been proposed for the transformation. Continued decomposition of the supersaturated solution occurs by the formation of a nonequilibrium phase denoted b› and a solid solution with less Mg content than the equilibrium, and then the formation of the equilibrium b phase. By rapid quenching techniques, the solubility of Mg in (Al) can be extended significantly beyond the equilibrium maximum solid solubility. [64Luo] extended the solid solubility to 36.8 at.% Mg; in a 40 at.% Mg alloy, the b phase was obtained. [73Gud] solidified alloys of composition 25 to 55 at.% Mg at cooling rates ranging from 102 to 108 C/s. At the lower cooling rates, b, g›, and g were formed; at higher cooling rates, a new phase, denoted f, was observed. [ 78Sur], using a "liquisol" quench, found that a metastable solid solution and a metastable phase appeared in a 30 at.% Mg alloy. Based on the structure, the new phase was identified as having the stoichiometry Al2Mg. [78Pre] found only a, g›, or g in splat-cooled specimens of composition between 0 and 63 at.% Mg, and no b or R phase. Specimens were fully (Al) up to 38.35 at.% Mg, beyond which the g› phase appeared. 33Sch: E. Schmid and G. Siebel, Z. Phys., 85, 37-41 (1933) in German. 35Hau: J.L. Haughton and R.J.M. Payne, J. Inst. Met., 57, 287-298 (1935). 35Zak: M.I. Zakharowa and W.K. Tschikin, Z. Phys., 95, 769-774 (1935) in German. 38Hum: W. Hume-Rothery and G.V. Raynor, J. Inst. Met., 63, 201-226 (1938). 38Kur: N.S. Kurnakov and V.I. Micheeva, Izv. Sekt. Fiz-Khim. Anal., 10, 37-66 ( 1938) in Russian. 39Sie: G. Siebel and H. Vosskuehler, Z. Metallkd., 31(12), 359-362 (1939) in German. 45But: E. Butchers and W. Hume-Rothery, J. Inst. Met., 71, 291-311 (1945). 64Luo: H.L. Luo, C.C. Chao, and P. Duwez, Trans. AIME, 230, 1488-1490 (1964). 70Ban: J. Bandyopadhyay and K.P. Gupta, Trans. Indian Inst. Met., 23(4), 65-70 (1970). 73Gud: V.N. Gudzenko and A.F. Polesya, Izv. V.U.Z. Tsvetn. Met., (4), 144-148 ( 1973). 78Pre: B. Predel and K. Hulse, Z. Metallkd., 69(10), 661-666 (1978) in German. 78Sur: C. Suryanarayana, S.K. Tiwari, and T.R. Anantharaman, Z. Metallkd., 69, 155-156 (1978). 79Sti: W. Stiller and H. Hoffmeister, Z. Metallkd., 70(12), 817-824 (1979). Published in Phase Diagrams of Binary Magnesium Alloys, 1988, and Bull. Alloy Phase Diagrams, 3(1), Jun 1982. Complete evaluation contains 4 figures, 15 tables, and 112 references. Special Points of the Al-Mg System
